6th Annual Pharma Pricing, Reimbursement & Market Access 2022
WHY SHOULD YOU ATTEND?
Get more from the event, with a broader scope bringing the whole communications value chain together. Enjoy and make the best out of our dedicated networking time, meet the leading international vendors showcasing the products of tomorrow in the co-located exhibition. Expand your knowledge of the latest business models and strategies in the high-level conference.
Event Speakers
RICHARD LINER
Bayer

RICHARD LINER
Bayer
RICHARD LINER, Senior Assistant General Counsel, Bayer
Richard Liner is Senior Assistant General Counsel for Bayer’sPharmaceuticals Division. He advises on a broad spectrum of complex fraud and abuse and compliance issues, including anti-kickback, anti-corruption, off-label promotion and HIPAA privacy. He routinely works on the development of policies, procedures and guidance for the Bayer pharmaceutical business and manages both external and internal compliance investigations. Richard graduated from Boston University’s School of Law with a concentration in healthcare law and received a Master’s degree in Public Health from The Harvard School of Public Health.
MARK MYERS
US Dept. of Veterans Affairs

MARK MYERS
US Dept. of Veterans Affairs
MARK MYERS, Director, Healthcare Resources Division, Office of Contract Review & Inspector General, United States Department of Veterans Affairs
JOLIE APICELLA
EDNY

JOLIE APICELLA
EDNY
JOLIE APICELLA, Assistant, U.S. Attorney’s Office, EDNY
JOLIE APICELLA is an Assistant United States Attorney and Chief of Civil Health Care Fraud in the Eastern District of New York. Since joining the USAO-EDNY in 2013, Jolie has specialized in affirmative civil litigation and successfully handled a range of complex health care fraud, financial, environmental and civil rights cases. In her role as Chief of Health Care Fraud, Jolie supervises over 20 AUSAs on investigations and litigation involving federal health care programs, ranging from cases against large pharmaceutical companies and hospitals to individual providers. She also carries her own docket of healthcare fraud cases.
RAHUL GOYAL
UnitedHealth Group

GREG APOSTOL
Alkermes

BOXIONG TANG
BeiGene

CHARLES BROWN
Novartis Gene Therapies

CHARLES BROWN
Novartis Gene Therapies
CHARLES BROWN, Director, Global Pricing and Contracting, Global Value & Access, Novartis Gene Therapies
Charles joined the Novartis Gene Therapies in 2021, as global director of pricing and contracting, leading access for patients with spinal muscalar atrophy. Previously, Charles headed pricing and access capabilities with the Global Novartis Oncology team, moving from Canada to the New Jersey Campus of Novartis Oncology. In this role, Charles led pricing and access strategies that have improved patient access to Novartis Oncology brands in Canada and Latin America. Before moving to the US, Charles was a member of the Canadian Novartis Oncology Value & Access team. In this role, Charles was driven to ensure oncology patients have access to the right medicine at the right time.
Charles has over 25 years of experience in the pharmaceutical Industry. He has also held positions in Canada as Health Policy and Patient Access Manager, Director of sales, Product Manager, and Manager for New Product Development.
Charles has a Bachelor of Arts in Economics from Simon Fraser University, as well as a Master of Business Administration from Royal Roads University, both in British Columbia, Canada. Charles enjoys reading and has a passion for rugby, cycling and travel.
KEITH WHITE
Amylyx Pharmaceuticals

LOUISE ST-ONGE
Mitsubishi Tanabe Pharma (Canada)

LOUISE ST-ONGE
Mitsubishi Tanabe Pharma (Canada)
LOUISE ST-ONGE, Head of Pricing, Market Access & Government Affairs, Mitsubishi Tanabe Pharma (Canada)
Louise St-Onge is the Head of Pricing, Market Access & Government Affairs at Mitsubishi Tanabe Pharma Canada. She is an accomplished Pharma Leader with >25 years executive experience and track record of achievements in Specialty Pharma, Oncology & Rare Diseases. Louise brings unique perspectives gained from her broad experience in Sales, Marketing and Patient Access, including in setting up Canadian Operations. She is an inspiring, change agent leader with strong ability to solve complex issues and certificated from the admired Bioinnovations/CCL Executive Leadership Program. She holds several awards for superior contribution to organizations and was nominated for prestigious awards in Health: Ernst & Young entrepreneurship, OCTAS for Techno Innovation.
ALAN POLNARIEV
Astrazeneca

STUART LEVINE
Chicago Pacific Founders

STUART LEVINE
Chicago Pacific Founders
STUART LEVINE, Partner, Chicago Pacific Founders
Founder, agilon health, Google Health, Director, Asst Clinical Professor, Internal Medicine/Psychiatry, UCLA & Stanford Schools of Medicine
PAUL E. F
ATRIO Health Plans

PAUL E. F
ATRIO Health Plans
PAUL E. FRIEDMAN, Medical Director, ATRIO Health Plans
Initially from New York, Dr. Paul Friedman completed a residency in Internal Medicine at Winthrop University Hospital before moving to California where he was a fellow in nephrology at L.A. County USC Medical Center. He practiced Internal Medicine, Nephrology and Long Term Care medicine for about 15 years after which time he obtained a Master's degree in Business Administration from UCLA. Since that time he has worked as a Medical Director for Health Plans, IPA's, and Medical Groups, focusing on Utilization Review and Case Management. He is currently working for Atrio Health Plans, an organization managing Medicare Advantage Plans in southern Oregon and Nevada. He is certified by the American Board of Internal Medicine and the American Board of Quality Assurance and Utilization Review Physicians.
HEATHER L F
Equideum Health

HEATHER L F
Equideum Health
HEATHER LEIGH FLANNERY, Founder & CEO, Equideum Health
Heather Leigh Flannery is the Founder and Chief Executive Officer of Equideum Health, a spin-off from ConsenSys AG effective March 2nd, 2020. ConsenSys Health rebranded to Equideum Health on January 18th, 2022. Equideum Health is a ConsenSys partner and a ConsenSys Mesh portfolio company. Heather is Vice Chair of the IEEE SA Open P2418.6 Standards Development Working Group (blockchain in healthcare and life sciences), has served as FY19 and FY20 Co-Chair and FY21 Chair of the global HIMSS Blockchain in Healthcare Task Force, chairs the Healthcare Interest Group at the Enterprise Ethereum Alliance (EEA), and is an Associate Editor of the peer-reviewed journal, Frontiers Blockchain for Science. Ms. Flannery is an active consultant, advisor, and keynote speaker.
Prior to ConsenSys, she founded and led Obesity Prevention, Policy, and Management, Inc. ("Obesity PPM"), an innovative provider of disease management, population health, research administration, and information technology managed services for health systems in the Americas, and an early adopter of blockchain technology.
Ms. Flannery has driven business model innovation via technology early adoption throughout her 28-year career as an entrepreneur, technologist, and strategist. She has consulted in the public sector in context of international development, bringing a global health perspective to her work. Focusing in the health sector since 2006, Ms. Flannery is a broad, lateral thinker who applies complex adaptive systems thinking to make progress against macroscopic challenges.
Due to her personal life experience, Ms. Flannery is intrinsically motivated to impact population health and economic prosperity. She builds mission-driven organizations aligned by shared values: making measurable societal contributions, creating economically sustainable interventions, and prioritizing diversity and inclusion
ROBERT P
Global Healthy Living Foundation

ROBERT P
Global Healthy Living Foundation
ROBERT POPOVIAN, Chief Science Policy Officer, Global Healthy Living Foundation
Dr. Robert Popovian is the Founder of the strategic consulting firm Conquest Advisors. He also serves as Chief Science Policy Officer at the Global Healthy Living Foundation, Vice-President, Health Economics and Policy for Equideum Health, Senior Healthy Policy Fellow at the Progressive Policy Institute and Visiting Health Policy Fellow at the Pioneer Institute. He previously served as Vice President, U.S. Government Relations at Pfizer.
One of the country’s foremost experts on every significant facet of biopharmaceuticals and the healthcare industry, he is a recognized authority on health economics, policy, government relations, medical affairs, and strategic planning.
Dr. Popovian has published extensively and referenced on the impact of biopharmaceuticals and health policies on costs and clinical outcomes in the most prominent medical sources and media publications, including the Clinical Economics and Outcomes Research, The Oncologist, Journal of Vaccines and Vaccinations, Health Science Journal, USA Today, Washington Examiner, Managed Healthcare Executive and The Hill. He is also a sought-after speaker and has been invited to provide detailed presentations, briefings, and expert reviews for the U.S. Congress, dozens of state legislatures, and at conferences and medical symposiums throughout the country and around the world.
Dr. Popovian is one of the select few researchers to study and publish both clinical and policy-related economic analysis as well as empirical data regarding emerging payment models in the U.S. healthcare system and for biopharmaceutical reimbursement. His insight and analysis also led to one of the first inclusions of health outcomes data regarding a biopharmaceutical labeled indication.
As an advisor, Dr. Popovian serves on the Board of Councilors of University of Southern California School of Pharmacy.
Dr. Popovian completed his Doctorate in Pharmacy and Master of Science in Pharmaceutical Economics and Policy degrees at the University of Southern California with honors. He has also completed a residency in Pharmacy Practice/Adult Internal Medicine and Infectious Diseases at the Los Angeles County-USC Hospital and a fellowship in Pharmaceutical Economics and Policy at USC.
Robert and his family have residences in Los Angeles, CA, and Washington, DC.
Email: rpopovian@gmail.com
LinkedIn: https://www.linkedin.com/in/robert-popovian/
Website: http://conquest-advisors.com/
Podcast: Healthcare Matters
Twitter: @PopovianPharmD
AYESHA AZAM
Patient Access Network Foundation

AYESHA AZAM
Patient Access Network Foundation
AYESHA AZAM, Vice President of Medical Affairs, Patient Access Network (PAN) Foundation
PETER B
Deallus

PETER B
Deallus
PETER BARSCHDORFF, Vice President, Deallus
Peter Barschdorff is a management consultant and corporate executive with over 20 years of experience, including at Deloitte, Bayer, and GSK. In his role as Vice President and Head of U.S. Consulting at Deallus he oversees partnerships with some of the world’s top pharmaceutical companies, ranging from complex and large-scale disease state monitoring and landscape assessments to commercial competitor analyses as well as simulation and strategy development. Trained a defense analyst, he started his consulting career through a plethora of managed market studies for top U.S. pharma's, across several key therapeutic areas, and still today gets involved in value solution and market access related client priorities. During his industry appointments he lead teams charged with, i.e.., the development of an AI engine to optimize patient access and Rx compliance, evaluation of patient pathways, segmentation of IDNs, and with health plan influence mapping vis-à-vis providers. Peter is based in New York (NY) and holds a Ph.D. from the Free University in Berlin and an MBA from Columbia Business School.
LinkedIn tag: https://www.linkedin.com/in/barschdorff/
VIDYA B D
Keen Access Insights

VIDYA B D
Keen Access Insights
VIDYA BREEVELD–DWARKASING, Owner, Keen Access Insights (Former Head of Public Affairs & Market Access, Sanofi Pasteur)
After 14 years in Pharma market access and public affairs and a previous 10 years in academics, Vidya Breeveld-Dwarkasing has decided to combine her strong science background with her helicopter view of the complex market access environment into Keen Access Insights. Keen Access Insights is a market access consultancy that helps Biopharma, Biotech and MedTech to address their market access and launch challenges by equipping them to find pragmatic solutions for complex problems. Next to that - driven by the urgency to help solve the geographical inequality of access to orphan medicines in Europe - Vidya has founded Transmineo, a startup company that uses modern digital and information technologies to set up a smart organization model to be offered as a service to companies to commercialize their orphan medicines in Europe in a highly cost-efficient way, while demonstrating value and addressing payer concerns. Vidya likes to challenge the existing status quo with the aim to stimulate a change for the positive.
REED STEPHENS
Winston & Strawn

REED STEPHENS
Winston & Strawn
REED STEPHENS, Partner, Winston & Strawn
T. Reed Stephens is a partner in the White Collar Defense Practice Group of Winston & Strawn’s Washington, D.C. Office. He is the head of the firm’s Healthcare/FDA Practice. Reed’s work has taken him to Europe and Africa, among other destinations, to providehishigh stakes risk mitigation and compliance advisory practice to clients.
As a trial attorney with the United States Department of Justice from 1995 to 2003, Reed broke new ground as lead counsel in various fraud matters involving the life sciences industry ushering in an era of regulatory compliance for the industry earning him a Civil Division Special Commendation Award in 2002. Since leaving the DOJ, Reed has been advising and defending global pharmaceutical companies, hospital systems, health plans, and other global corporations on False Claims Act litigation (including precedent-setting Stark Law litigation), health care fraud & abuse issues, FDA regulatory, Congressional investigations into drug pricing strategies launched by both the Senate and House of Representatives, government contracts (including voluntary disclosures of billing irregularities), foreign & domestic bribery statutes (including the Foreign Corrupt Practices Act), merger and acquisition due diligence risk, and individual white collar criminal exposure.
Reed earned his undergraduate and law degrees from Princeton University and Stanford Law School, respectively. Reed’s honors includeLaw360 Life Sciences Editorial Advisory Board 2022;Chambers USA Ranking in Health Care2008 - 2014; Benchmark Litigation, Local Litigation Star 2017; LMG Life Sciences, Life Sciences Star, 2013 - 2019; Washingtonian Magazine, Washington's Best Lawyers 2013; On Being A Black Lawyer’s Nation’s Top Lobbyists And Influencers, 2014 and 2019, 2020; and Savoy Magazine’s Top Black Lawyers of 2015.
MARGAUX J. H
ROPES & GRAY

MARGAUX J. H
ROPES & GRAY
MARGAUX J. HALL, Partner, ROPES & GRAY
Practice
Margaux Hall is a partner in the firm’s nationally recognized health care practice. Margaux is a leading lawyer in drug pricing, market access and value-based arrangements. She brings to clients an understanding of the transformative legal and policy issues and market changes affecting pricing and access to drug and vaccine products, including in the wake of the COVID-19 pandemic.
Named a Law360 “Rising Star” for health care in 2019, Margaux provides sophisticated regulatory and strategic business counsel to clients – including pharmaceutical manufacturers, investors, and others – on matters involving coverage and reimbursement under Medicare Advantage-Part D, Medicaid, the Federal Supply Schedule, and other government programs; innovative contracting and payment arrangements under government and commercial contracts; and price reporting obligations under federal and state laws.
Margaux’s deep knowledge of the pharmaceutical supply chain also is invaluable in major life sciences transactions. Investors and others turn to Margaux to assist them in assessing and evaluating their investments in pharmaceutical products, specialty and long-term care pharmacies, pharmacy benefit managers, distributors, group purchasing organizations, and other companies providing services to entities in the supply chain.
Margaux has dedicated her career to understanding health care in the U.S. and worldwide; after graduating from Harvard Law School, she pursued a Fulbright Fellowship to research health care law and health system reform in South Africa and then led similar research programs at the World Bank. Margaux also completed a post-graduate fellowship focusing on health law through Columbia Law School.
Margaux also has developed a robust practice with pro bono clients in the health care arena. As part of this, she has long represented Capital Clubhouse, a nonprofit organization serving people living with mental illness, during its creation of a community health center.
Honors & Awards
- Law360 Rising Star (2019)
- Fellow, Columbia Law School, focus on health care law (2012-2014)
- Fulbright Scholar, South Africa (2008-2009)
Memberships
- American Health Lawyers Association
- American Bar Association
FANTA W
Serrette Brown Research & Consulting

FANTA W
Serrette Brown Research & Consulting
FANTA WATERMAN, Managing Director, Serrette Brown Research & Consulting
Fanta Waterman PhD, MPH is an award-winning health services researcher who is passionate about making health literacy achievable for everyone, and investigating opportunities to improve representation and patient-centeredness in health research to improve the health and quality of life of patient populations. Dr. Waterman has held positions in local government agencies, consulting firms, non-profits, large manufacturers, and start-ups. With numerous publications investigating patient behaviors and health outcomes, Dr. Waterman is also a seasoned lecturer, having taught as an adjunct Associate Professor within the City University of New York from 2008 to 2013.
FABRIZIO G
Universities of Rome and Ferrara

FABRIZIO G
Universities of Rome and Ferrara
FABRIZIO GIANFRATE, Professor of Health Economics and Outcome Research, Universities of Rome and Ferrara
TED KARNEZIS
Karnezis Consulting

TED KARNEZIS
Karnezis Consulting
TED KARNEZIS, Owner, Karnezis Consulting
Ted Karnezis - Principal/Owner of Karnezis Consulting LLC, established in October of 2020.
Ted’s experience spans a decade at the Department of Veteran’s Affairs Pharmacy Benefits Management working with the pharmaceutical industry across various aspects of Government pricing, FSS contracts and the Veteran’s Healthcare Act of 1992 (VHCA) compliance. Ted has worked directly with the Offices of Inspector General (OIG), assisting with confidential matters in pre & post contract award reviews and pricing calculation methodologies.
While at the VA, Ted’s primary responsibility was to manage the Veteran’s Healthcare Act of 1992 (VHCA) and maintaining the Federal Supply Schedule (FSS) contract pricing. Known informally to industry as “Public Law Season”, Ted was an integral part of ensuring the Public Law season ran smoothly and streamlined the annual reporting process and tightened program requirements.
Karnezis Consulting has provided solutions to pharma industry since October of 2020 assisting with Government Pricing & Calculations compliance.
Web: www.karnezisconsulting.com
LinkedIn: https://www.linkedin.com/in/ted-karnezis-chicago/
CHRISTOPHER H. S
LATHAM & WATKINS

“Critical guide for successfully identifying your pricing, reimbursement and market access strategies”
Event Schedule
Learn, meet and network with your conference colleagues.
09:30 – Chairperson Opening Remarks
PETER BARSCHDORFF, Vice President, Deallus
09:40 – Real World Evidence (RWE) in the reimbursement decision-making in Canada
- Overview of the reimbursement landscape in Canada
- How RWE can support the decision-making for the different payers
- Considerations for leveraging RWE across the product life cycle
- Role of Patient Support Programs
LOUISE ST-ONGE, Market Access & Government Affairs, Mitsubishi Tanabe Pharma (Canada)
10:20 – Application of evidence based medicine and value based contracting to insure improved patient quality, eliminate patient and physician abrasion and lower total cost of care. The role of the:
- Health Plan
- PBM
- Delegated full risk medical group
- Physicians
- Patients and family
How to organize this and what are the levers and expected results to achieve success
STUART LEVINE, Partner, Chicago Pacific Founders
PAUL E. FRIEDMAN, Medical Director, ATRIO Health Plans
11:00 – Morning Coffee/Tea & Networking
CHALLENGES & OPPORTUNITIES
11:20 – Keynote Panel Discussion: Pharma pricing and market access in the US: concepts, components, and future
- New developments in pharma pricing and market access in the US
- Staying ahead in the race - Update on pricing and market access in USA, EU & RoW –
- Evidence driven pricing and reimbursement strategy
- Market access scenario in developed markets versus emerging markets
- Returning to the new normal: HTA & Reimbursement challenges in a post-pandemic world
- Innovator strategy and investor due diligence
- Value Assessment, navigating the global landscape
Moderator:
RAHUL GOYAL, Senior Director, UnitedHealth Group
Panellists:
MARTIN ROST, Senior Director, Market Access (Global), Pfizer
MICHAEL THOMPSON, President & CEO, National Alliance of Healthcare Purchaser Coalitions
JOLIE APICELLA, Assistant, U.S. Attorney’s Office, EDNY
KEITH WHIT, Head, Global Market Access, Amylyx Pharmaceuticals
12:10 – Implementing a Pricing and Market Access Strategy.
- The importance of providing the key payer requirements.
- The value story that payers want in order not to view a pharmaceutical as a commodity.
- How a persuasive value proposition for a pharmaceutical is structured and common gaps in the evidence supporting a value proposition.
- Overcoming the problem of not acting early enough to develop pricing power.
- Patient assistance programs: do these augment the perceived value of the pharmaceutical or are they just the cost of doing business?
ANDREW THORRENS, VP / Head of Market Access (US) and Reimbursement, ImprimisRx
12:50 - Networking luncheon
REGULATION OVERVIEW & UPDATE
13:50 – Keynote Panel Discussion: Regulatory updates and development
- Politics, Payers and Pandemonium: An insight into the future US pricing and market access environment
- Complex regulatory and reimbursement pathways, varied evidence requirements and long procedural timelines pose risk to successful access and launch
- Policy issues that affect pricing and reimbursements
- Understanding the effects of forthcoming regulatory changes on your access, pricing and reimbursement efforts
- Possible increased synergy between HTA and regulatory agencies – Opportunity or challenge for medical devices?
- How are we to be working with payers and governments?
- Gain clarity on issues of standards for licensure and indication extrapolation
- Emerging trends and vision for future
Moderator:
PETER BARSCHDORFF, Vice President, Deallus
Panellists:
RICHARD LINER, Senior Assistant General Counsel, Bayer
ROBERT POPOVIAN, Chief Science Policy Officer, Global Healthy Living Foundation
REED STEPHENS, Partner, Winston & Strawn
TECHNOLOGY – SHAPING THE FUTURE
14:40 - Blockchain in the Pharmaceutical Industry: a Primer for Interpreting New Market Access Dynamics from Precision Medicine to Digital Therapeutics to Health Equity.
HEATHER LEIGH FLANNERY, Founder & CEO, Equideum Health
15:10 – Afternoon Tea/Coffee & Networking
15:30 - The Intersection of Blockchain and Biopharmaceutical Access, Economics and Research
- The impact of blockchain currently on the US biopharmaceutical supply chain
- Current endeavors in implementing blockchain in biopharmaceutical research and development
- Future implications of blockchain concerning biopharmaceutical payment models
- Engagement of patients through blockchain technology
HEATHER LEIGH FLANNERY, Founder & CEO, Equideum Health
ROBERT POPOVIAN, Chief Science Policy Officer, Global Healthy Living Foundation
HTA
16:10 – HTA and decision making in the reimbursement of medicines
- New drugs are failing to gain reimbursement from payers/HTAs at an alarming rate, despite being approved by regulators
- Market access, pricing and reimbursement strategy getting in the way of launch success
- Maximizing access to drugs – debating future of global healthcare systems
- Health policy development using outcomes research issues
- Is there room for innovation in HTA & reimbursement schemes – and what critical capabilities are needed?
VIDYA BREEVELD–DWARKASING, Owner, Keen Access Insights (Former Head of Public Affairs & Market Access, Sanofi Pasteur)
16:50 - 17:00 – Chairperson’s closing remarks and end of conference day 01
17:00 – 18:00 – Networking Drinks
09:30 – Chairperson Opening Remarks
PETER BARSCHDORFF, Vice President, Deallus
09:40 – Importance of Clinical and Economic Evidence to Support Product Reimbursement and HTA Needs
- Evidence needs by major HTA agencies,
- Generate scientific evidence in clinical development program,
- Comparative data analysis with standard of care,
- Head-to-head studies and indirect treatment comparisons
- Role of real-world evidence in regulatory and reimbursement decisions
- Health economic modelling and economic evaluation,
- Including clinical and economic evidence in economic modelling
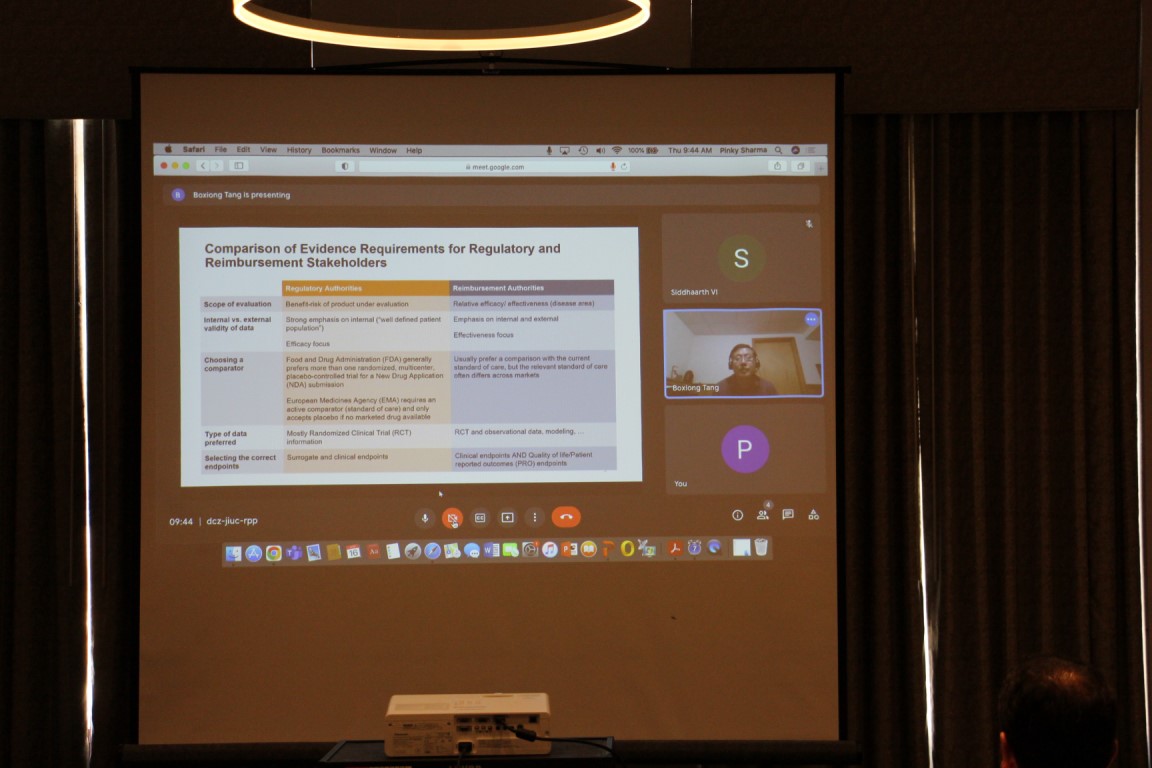
BOXIONG TANG, Executive Director, HEOR, Global Medical Affairs, BeiGene
10:20 – AI: HealthCare’s gateway to the future
ALAN POLNARIEV, Senior Medical Science Liaison, Astrazeneca
11:00 – Morning Coffee/Tea & Networking
PATIENT FOCUS
11:20 – Panel Discussion: Prioritising Patients! - Adding value through an innovative patient-centred approach
- The Patient, Payer and Provider
- The drive for better patient centricity from a pharma perspective
- How can HTA and Regulatory Agencies better meet the needs of patients by ensuring the patient perspective is held paramount
- Opportunities for meaningfully engaging patients in medicines research, development & delivery
- Understanding their unmet needs
- Best practices for collecting and incorporating patient insights
- Driving next generation patient-centric platforms and engagement
- Bridging communication gap between medical doctors and everyday patients
Moderator:
MELVA COVINGTON, Senior Vice President, Research and Patient Outcomes, Curio Digital Therapeutics
Panellists:
AYESHA AZAM, Vice President of Medical Affairs, Patient Access Network (PAN) Foundation
FANTA WATERMAN, Managing Director, Serrette Brown Research & Consulting
CHRISTOPHER H. SCHOTT, Partner, LATHAM & WATKINS
WILLIAM SARRAILLE, Partner, Sidley Austin
12:00– Requirements of The Veterans Healthcare Act of 1992 for Pharmaceutical Manufacturers
- Basic requirements to sell covered drugs to Federal marketplace
- What is a covered drug?
- What is the Federal Supply Schedule?
- Roadmap for Compliance
MARK MYERS, Director, Healthcare Resources Division, Office of Contract Review & Inspector General, United States Department of Veterans Affairs
12:40 - Networking luncheon
13:40 – Assessing and rewarding Innovation: the Italian AIFA algorithm
- The old and new algorithm
- Full or partial innovative status
- Driving criteria
- Access benefits
- Financial reward
FABRIZIO GIANFRATE, Professor of Health Economics and Outcome Research, Universities of Rome and Ferrara
14:20 - Employing predictive models to execute value-based pricing and contracting
- Predictive model methodologies
- Modeling for enriched targeting
- Establishing evidence-based metrics for pricing and contracting
EVERETT CROSLAND, Chief Commercial Officer, Cognito Therapeutics
15:00 – Afternoon Tea/Coffee & Networking
PAYERS – INDUSTRY - GOVERMENT
15:30 – Panel Discussion: Importance of working together: Stakeholders - Industry, Payers, Physicians and Patients
- The call for collaboration to achieve market access – who is calling and are payors truly willing?
- The New Normal - Pioneering Digital Stakeholder Engagement
- How can Pharma companies shake loose their negative reputation and become seen as partners in the healthcare system?
- Successful product launches benefit more than just the industry by working together from the start.
- Are there any stories of how collaborative partnerships to launch products in managed entry markets turned successful? What defines success and by whose definition?
- Barriers to market access and commercial success in emerging markets
Moderator:
VIDYA BREEVELD–DWARKASING, Owner, Keen Access Insights (Former Head of Public Affairs & Market Access, Sanofi Pasteur)
Panellists:
MELVA COVINGTON, Senior Vice President, Research and Patient Outcomes, Curio Digital Therapeutics
GREG APOSTOL, Vice President and Head of Market Access, Alkermes
CHARLES BROWN, Director, Global Pricing and Contracting, Global Value & Access, Novartis Gene Therapies
MARGAUX J. HALL, Partner, ROPES & GRAY
TED KARNEZIS, Owner, Karnezis Consulting
16:20 - 16:30 – Chairperson’s closing remarks and end of conference day 02
Event Sponsors & Partners
Virtue Insight
CONCEPTUALISED BY

Virtue Insight
CONCEPTUALISED BY
Virtue Insight equips business professionals around the world with the latest indepth industry knowledge and provides networking opportunities in the telecom, infrastructure and pharmaceutical industry. Our aim is to provide a platform to share knowledge and insights and provide our event attendees to network effectively and deliver maximum ROI by make new business alliances. We strive to produce high quality conferences which include the latest topics which are delivered by world class leaders of the industry.
Our motto is to offer our customers the expertise and connections for a profitable business. Our events encompass an optimum chance to gain maximum value in terms of networking and an opportunity to sponsor and exhibit to attract new business alliances.
Testimonials

Rare Advocacy Movement Co-Founder

Lakehead University Associate Professor

BresMed Health Solution Principal Research Associate

BresMed Health Solutions Graduate Health Economist
Gallery
Get Involved
Speaking Opportunities
Fen Castro
fen@virtueinsight.co.in
+91 44 42108101
Sponsor / Exhibit / Delegate Bookings
Piyush Patel
piyush@virtueinsightevents.com
+44 20 3509 3779